Clinical Study
As an international team, the clinical study team is committed to providing integrated clinical services and solutions for our customers’ products, supporting companies on marketing their products efficiently in China and overseas. The team has been involved in clinical studies of nearly 100 products.
Service Capability
We are able to provide clinical study services including BE test, PK/PD test, I-IV clinical test and post-marketing clinical study,and build a one-stop full-process service from clinical development strategy,study protocol design, clinical project management, clinical monitoring, quality control, pharmacovigilance,data management and statistical analysis, biological sample analysis and summary report writing to project registration and declaration.We boast rich clinical resources in China and India, and have extensive in the development and registration of dual submission products in China and the United States.
Pharmacovigilance
Establishment of safety management plan (SMP); Security database construction; Processing of individual case safety report (ICSR); Writing of Development Safety Update Report(DSUR).
Data management and statistical analysis
Database design, establishment, testing and maintenance;Case report form(CRF、eCRF) design;Clinical test design and sample size calculation;Establish a data set conforming to CDISC SDTM/ADaM standard.
Medical service
Clinical strategy;Protocol design;Medical document writing;Medical monitoring.
Clinical operation management
Project management;Project monitoring and quality control;Application submission of Human Genetic Resource Administration of China(HGRAC);Third-party audit and Self-inspection before NMPA verification.
Biological sample analysis
Biological analysis of chemical drugs and metabolites;Clinical sample collection manual and sample management;PK/PD analysis and modeling;Biological analysis laboratory and project audit.
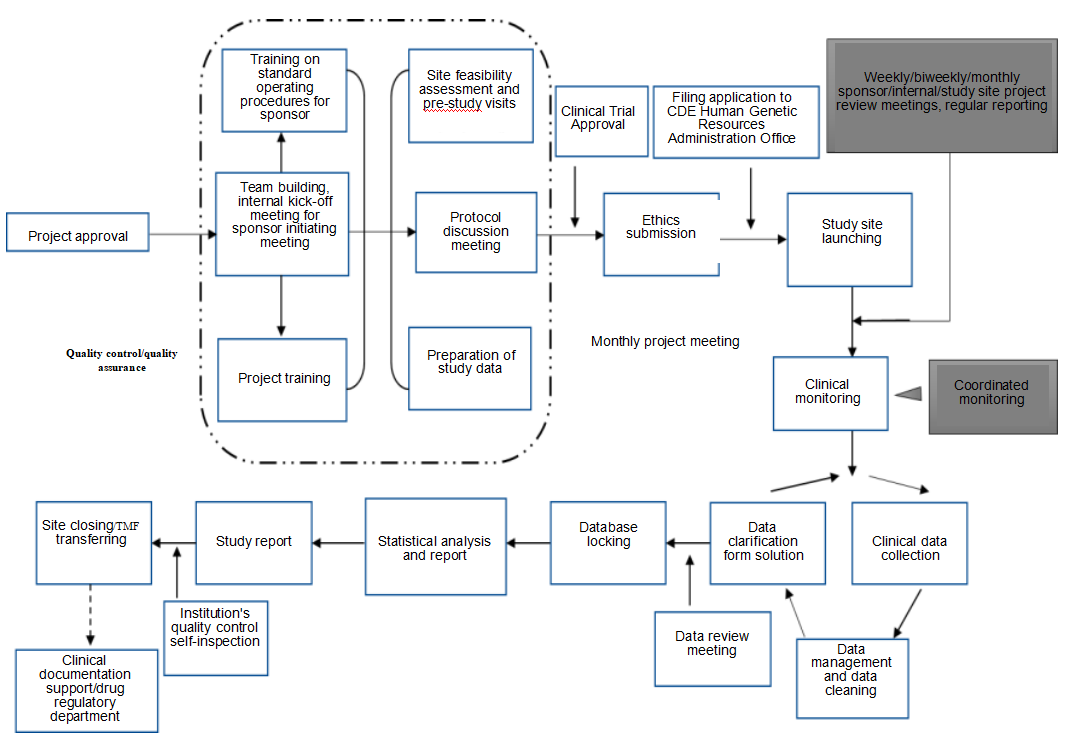
Service Process